Most Popular
Hand sanitizer alcohol content more than 60%
Experts pointed out that the alcohol content of less than 60% of hand sanitizer can kill bacteria, but currently on ... ...
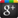


The molecular structure of soap
Rated: 
 , 0 Comments
, 0 Comments
Total hits: 1802
Posted on: 01/28/16
Soap molecules structure can be divided into two parts.End is to take charge of polarity - the COO (hydrophilic), on the other side for non-polar chain (oil wet parts).Soap can break the water's surface tension, when soap molecules in the water, has the polarity hydrophilic parts, will destroy the attraction between water molecules and to lower the surface tension of the water, the water molecules on both land distribution on the surface with clean clothing or skin.Oil wet parts of the soap, grease, soluble in water and hydrophilic parts, this combination after stirring to form smaller oil droplets, the surface is full of hydrophilic parts of the soap, and won't back together into a big oil.This process (also known as emulsification) repeat many times, all the oil will become very small oil droplets dissolve in the water, can be easily rinsed clean.
We are soap manufacturers, we producting all kinds of hand washing liquids,and sell it.
Comments
There are still no comments posted ...
Rate and post your comment